Abstract
Recent reports suggest that survivors of hematopoietic cell transplantation (HCT) exhibit clinical features consistent with accelerated aging including increased incidence of endocrinological, pulmonary and cardiovascular morbidity, other severe chronic disorders and excess of secondary malignancies. In addition, high incidence of frailty - a physiologic measure of aging, was documented in childhood cancer survivors. Data from additional studies suggest that cytotoxic therapy triggers an acceleration of the aging process whose likely mechanism may be cellular senescence. In order to corroborate the physiological and epidemiological data, more accurate biological markers of senescence should be assessed. Indeed, several studies, including from our group, reported on telomere shortening after HCT. Other studies showed elevation of inflammatory biomarkers, those being associated with the aging process. The accuracy of these biological markers is disputable due to inherent problems in their interpretation. Recently, several more accurate biomarkers of cellular age have been reported. The most promising is the DNA methylation status of several genomic loci, which can determine the cellular age with accuracy of 1.5 to 3 years. Additional markers, such as p16ink4A and IL-6 concentrations in serum are also considered as reliable markers of age.
The present study assessed the hematopoietic and non-hematopoietic systems aging trajectory after allogeneic HCT. We took advantage of being able to assess quantitatively the hematopoietic system comparing blood cells derived from one person (the donor) and residing in two different hosts (donor and recipient). The blood cells aging may be affected by replicative stress after HCT and therefore do not necessarily represent the whole body aging; therefore we measured the age of buccal epithelial cells of the transplant survivors as well. For cellular age determination we used DNA methylation in three sites, previously validated as an accurate age determination method, p16ink4A blood levels and serum IL-6 concentration. To the best of our knowledge, this is the first study employing these novel markers for assessment of accelerated aging in a clinical setting.
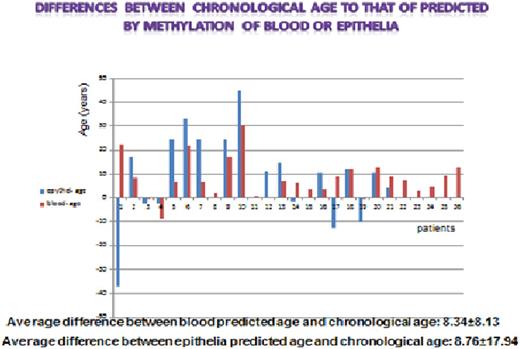
26 survivors of allogeneic HCT (median age, 48 years; range, 28 to 71) were evaluated at a median of 3.6 (range, 2.2 to 9) years after HCT. The cellular age of donor blood cells residing in the recipient was compared with the age of the blood cells residing in the respective donor and the cellular age of the patients' buccal epithelial cells was compared with their chronological age.
In 21 out of 26 patients the cellular age of donor blood cells residing in the patients was significantly higher than that of the respective cells residing in the donor as determined by DNA methylation. The mean excess aging of the cells was 8.3 (range -5 to 30) years and in most of the patients the cells were more than 10 years "older" than the donors' cells. The buccal epithelial cells predicted age was also significantly higher than the chronological age; the median excess of aging was 8.7 (range, -37 to 10.5) years. The two other biomarkers of aging: IL-6 and p16ink4A also demonstrated advanced age compared to the chronological age. There was no association between the magnitude of aging and previous chemotherapy or GVHD. Telomere length was not significantly shortened in the transplanted patients compared to their donors, consistent with previous reports demonstrating initial telomere shortening and eventual return to normal levels.
In conclusion, allogeneic stem cell transplantation is associated with significant accelerated aging of both of the hematopoietic and somatic systems.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal